Mycoplasma Pneumoniae Acidum nuclei
Product nomen
HWTS-RT124A-Congelo-siccata Mycoplasma Pneumoniae nuclei Acidum Detection Kit(Enzymatic Probe Isothermal amplification)
HWTS-RT129A-Mycoplasma Pneumoniae nuclei Acidum Detection Kit(Enzymatic Probe Isothermal amplification)
Testimonium
CE
Epidemiologia
Mycoplasma pneumoniae (MP) minimum est microorganismus prokaryoticus cum cella structura et nulla paries cellae inter bacteria et virus.MP maxime causat infectiones tractus respiratorii in hominibus, praesertim in pueris et iuvenibus.MP causare potest Mycoplasma hominis pneumonia, tractus respiratorii infectiones in pueris et pneumonia atypica.Signa clinica diversa sunt, plerumque tussis gravis, febris, frigora, capitis faucium, tractus respiratorii superioris infectio et bronchopneumonia frequentissima sunt.Nonnulli aegroti graves peripleumonia ex contagione tractus respiratorii superioris, et angustia respiratoriae graves vel etiam mors evenire possunt.MP est una e communibus et magnis pathogenis in communitate peripneumonia quaesita (CAP), ratio pro 10%-30% cap, et proportio 3-5 vicibus augeri potest cum MP praevalet.Nuper, proportio MP in CAP pathogens paulatim aucta est.Incidentia Mycoplasma pneumoniae infectio aucta est, et propter eius manifestationes non specificas clinicae facile confundi cum frigoribus bacterial et viralibus.Ergo mane detectio laboratorium magni momenti est pro diagnosi et curatione clinica.
Channel
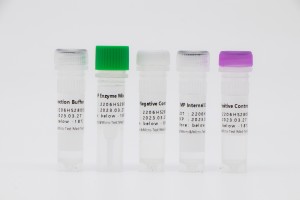
| FAM | MP Acidi nuclei |
| ROX | Internum Imperium |
Technical Parameters
| Repono | Liquid: ≤-18℃ In obscuro, Lyophilized: ≤30℃ In obscuro |
| Fasciae vita | Liquid: 9 menses, Lyophilized: 12 months |
| Specimen Type | faucium swab |
| Tt | ≤28 |
| CV | ≤10.0% |
| LoD | 2 Copia/μL |
| Specification | Nulla crux-reactividad cum aliis speciminibus respiratoriis ut Influenza A, Influenza B, Legionella pneumophila, Rickettsia Q febris, Chlamydia pneumoniae, Adenovirus, Virus Syncytialis respiratorii, Parainfluenza 1, 2, 3, virus Coxsackie, virus Echo, Metapneumovirus A1/A2/ B1/B2, Virus syncytialis respiratorii A/B, Coronavirus 229E/NL63/HKU1/OC43, Rhinovirus A/B/C, Virus Boca 1/2/3/4, Chlamydia trachomatis, adenovirus, etc. et genomicum humanum DNA. |
| Applicabilis Instrumenta | Acta Biosystems (VII)D Real-time PCR Systems SLAN ®-96P Real-time PCR Systems LightCycler® 480 Real-Time PCR systema Securus Amp Real-time Fluorescens Isothermal Detection System(HWTS1600) |
Opus O
Optio 1 .
Commendatur extraction reagens: Macro & Micro-test Viralis DNA/RNA Kit (HWTS-3001, HWTS-3004-32, HWTS-3004-48) et Macro & Micro-test Extractor Nucleici Acidi (HWTS-3006).
Optio II.
Commendatur extractio reagens: Acidum nuclei extractio seu Purificatio Kit (YD315-R) fabricata per Tiangen Biotech (Beijing) Co., Ltd.

.jpg)
-300x300.jpg)